March
2017
HYDROCARBON
ENGINEERING
58
including low energy density and infrastructure
requirements. Methanol to gasoline (MTG) technologies have
been tested in commercial situations, including at
ExxonMobil’s MTG plant in New Zealand in the 1980s.
Increasing restrictions on the amount of aromatics allowable
in fuels has become the major barrier to traditional MTG
processes, which make >25 vol% aromatic compounds.
Butane glut
Natural gas liquids (NGLs), which comprise mainly of
ethane, propane, butanes and natural gasoline, production
is expected to increase more than 40% over the next
five years. The increase will total approximately
950 000 bpd, with volumes reaching at least 3.1 million bpd
by 2017. While ethane and propane serve as feed for steam
crackers to make ethylene and propylene, butanes
(isobutane and n-butane) do not have a ready market
outlet. Butane is blended into gasoline in the winter but,
due to its high vapour pressure, it cannot be blended into
gasoline in the summer.
Alkylate demand
Tier 3 refers to a set of fuel and vehicle standards adopted
by the US Environmental Protection Agency (EPA) in 2014.
When implemented in 2017, the standards will immediately
reduce toxic air pollution from cars and trucks. Oil
companies must lower the sulfur content of gasoline,
making it cleaner to burn. Refiners have a limited number of
options to reduce gasoline sulfur levels to meet the new
10 ppm requirement. For Tier 3, removing the remaining,
more difficult sulfur molecules may lead to more significant
octane loss. Accordingly, alkylate has emerged as a
preferred gasoline blending component, as it contains no
sulfur, no olefins, no benzene and has a low vapour
pressure and high octane number. Alkylate is produced by
reacting isobutane with light olefins using liquid acids, and
currently US refineries produce 1.3 million bpd of alkylate
(20 billion gal./y). Unfortunately, for many refiners alkylate
production is limited due to a shortage of olefins.
Breakthrough technology
Alkylate, a mixture of isoparaffins, is produced by the
reaction of C
3
– C
5
olefins with isobutane. It is highly
valued as a gasoline blendstock because it has no olefins or
aromatics, while also having an ultra low sulfur content,
low vapour pressure and high research and motor octane
numbers. Unlike all other alkylation technologies, this new
technology developed by Exelus requires no olefin
feedstock. Instead, methanol and butanes are converted to
alkylate using a three step process, as illustrated in Figure 1.
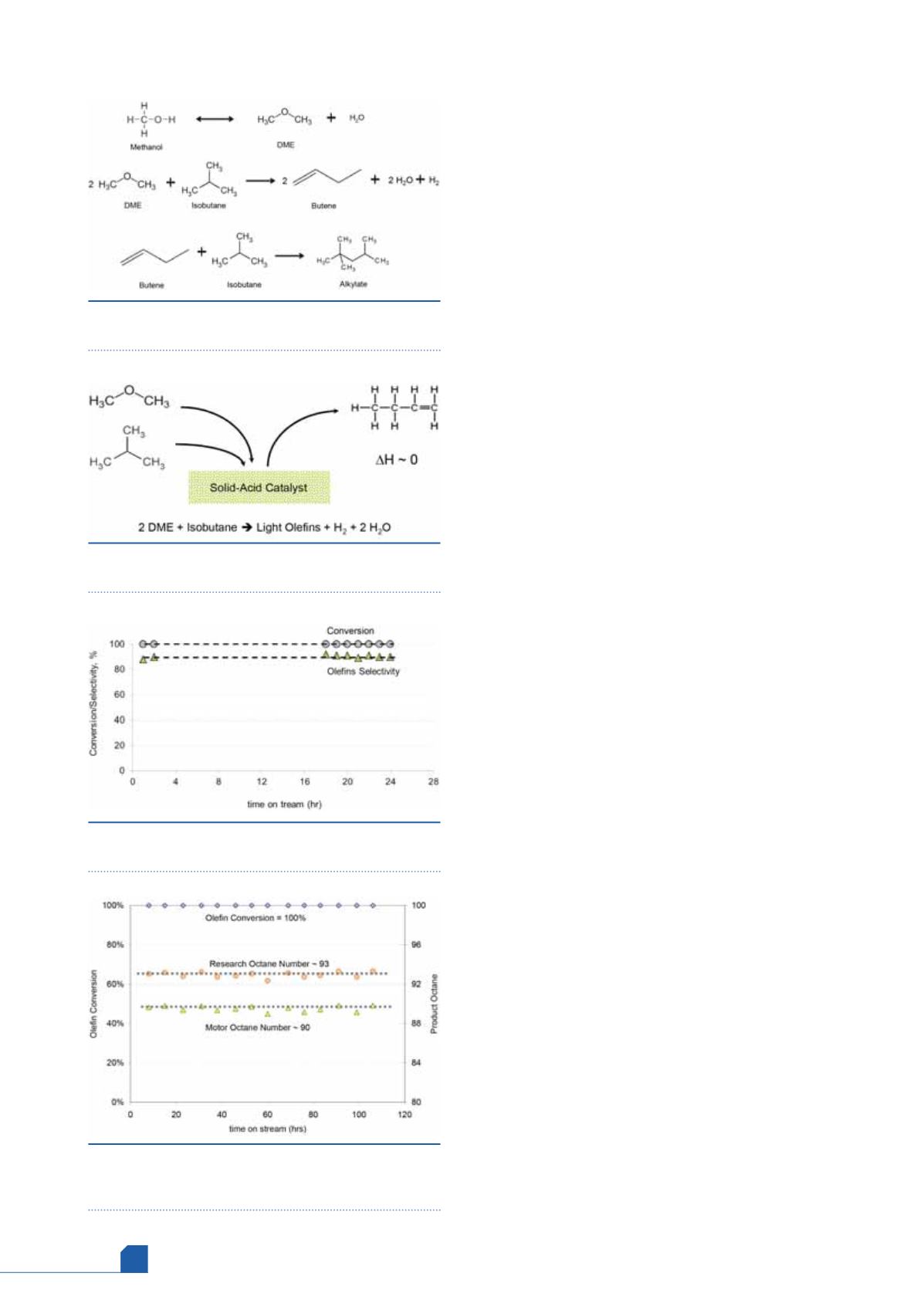
Step 1: conversion of methanol to dimethyl
ether
Methanol can be partially dehydrated to an equilibrium
mixture of dimethyl ether (DME), methanol and water over
a mild solid-acid catalyst (typically gamma-alumina). This
reaction is rapid, reversible and exothermic. This is a proven
technology and DME yields greater than 99% are reported.
In cases where the starting material is DME rather than
methanol, this first step can be omitted.
Figure 1.
Main reactions in the production of alkylate
from methanol and butanes.
Figure 2.
Unique solid-acid catalyst converts DME
and butanes simultaneously to light olefins.
Figure 3.
Time on stream performance of solid acid
catalyst for olefins production.
Figure 4.
ExSact-E catalyst produces high octane
alkylate from a mixture of light olefins: mainly
ethylene, propylene and butenes.








