March
2017
HYDROCARBON
ENGINEERING
66
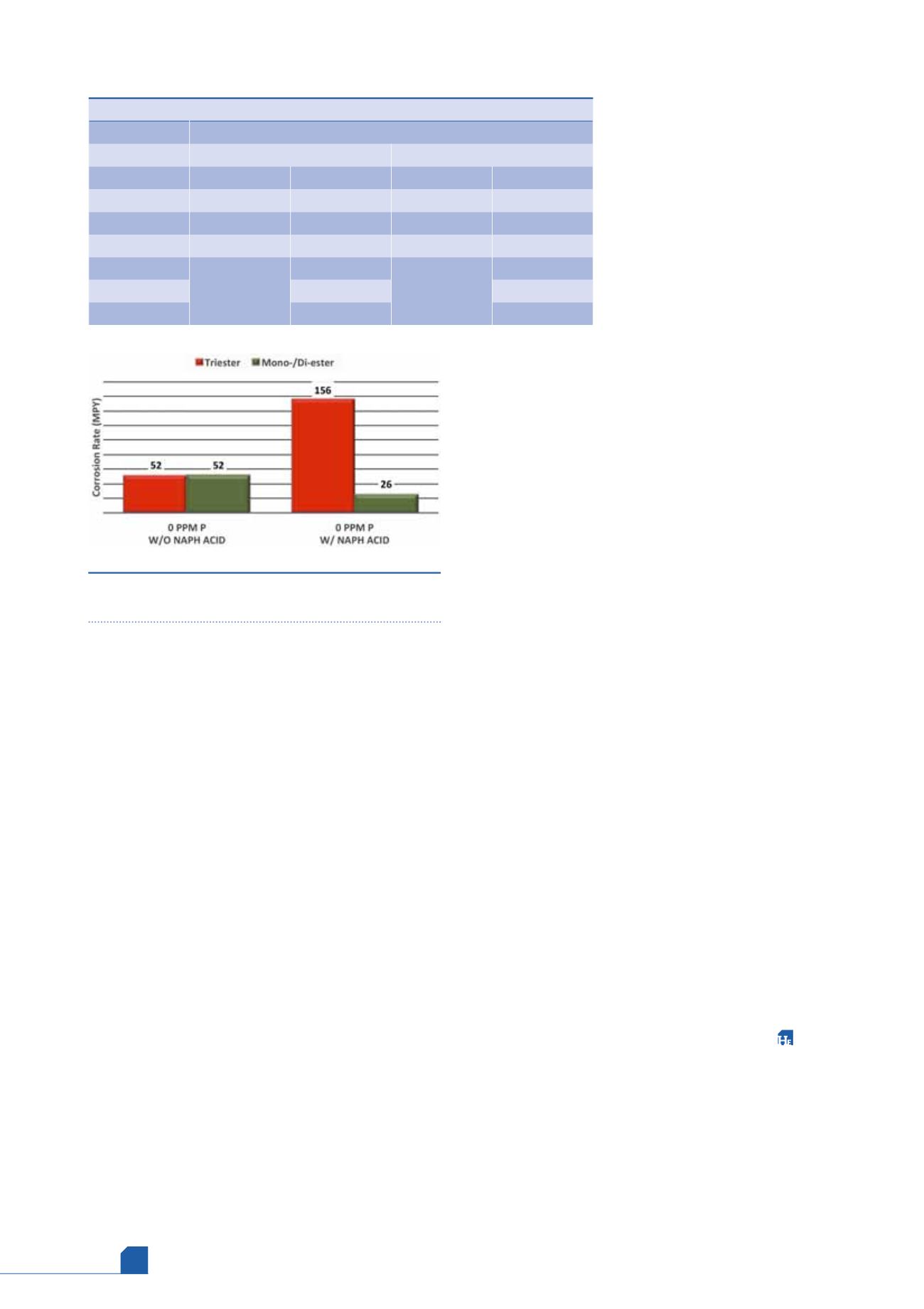
had similar corrosion inhibition properties when the
coupons were pre-passivated (Figure 4).
Simulating field conditions, the same experiment
was performed without pre-passivating the corrosion
coupons prior to testing in a fluid already containing
naphthenic acid. The results (shown in Figure 5) were
unexpected. The mono-/di-ester product afforded
almost 99% inhibition whereas the tri-ester product
resulted in a near 0% inhibition.
The above data clearly suggests that there is a
marked difference between the passivation layers that
are formed and this depends on the types of esters
that are in the presence of naphthenic acid. It is
expected that in the field, the passivation from the
tri-ester would be less likely to form under typical
process conditions.
To further investigate this, a dosage response test
was performed to determine if concentration could
have an effect on the ability of the esters to form
protective barriers. Table 2 shows the dosage response
required to achieve the same level of protection.
The test conditions used were the same as shown
in Figure 5, except for the dosage. Experiments were
conducted for both 4 and 12 hour durations. The
results, shown in Table 2, indicate that the amount of
the tri-ester required to provide equivalent corrosion
inhibition as mono-/di-ester would be a minimum of
2.5 times.
The data clearly shows that mono-/di-ester forms
a stronger barrier and is a more efficient corrosion
inhibitor compared to tri-ester in the presence of
naphthenic acid in simulated field
conditions.
Persistency of the
barrier layer
Persistency of the barrier layer is
important as the barriers have to
be able to exist during operating
conditions where the
replenishment of the inhibitor in
the process is not always perfectly
uniform. As such, a set of
experiments were conducted to
evaluate the persistency of the
barriers formed by mono-/di-ester and tri-ester in the
presence and in the absence of naphthenic acid. The
lubricant industry has reported that tri-ester forms a
strong physisorption film, whereas mono-/di-esters
chemically reacts and forms a strong chemical bond
with metal.
3
Chemically formed barriers such as this
should be more persistent than a physisorbed film.
In the first experiment, pre-passivation was carried
out, as in the previous experiments, without
naphthenic acid. The test was conducted for 12 hours,
5 TAN, 600 RPM at 653°F (345°C) with no additional
chemical dosage added. At the end of the
experiments, the coupons were measured for weight
loss.
In the second experiment, pre-passivation was
carried out as in the previous experiments, in the
presence of naphthenic acid with an approximate TAN
of 5. The rest of the procedure remained the same.
The results shown in Figure 6 demonstrate that
mono-/di-ester forms a stronger barrier (passivating
layer) in the presence of naphthenic acid compared to
tri-ester. The tri-ester was generally unable to form a
protective barrier in the presence of naphthenic acid
equivalent phosphorus dosage.
Summary
The use of phosphate esters-based inhibitors still
dominates the field of naphthenic acid corrosion
control by chemical additives. There are significant
differences in performance between the various esters
and this should be considered when selecting the type
to be used in the industry. The mono-/di-ester-based
additives demonstrate the most desirable
characteristics to control naphthenic acid attack in the
form of effective laydown efficiency and the formation
of a tenacious, persistent passivation barrier on the
metal surfaces found in refinery process units.
References
1. BABAIAN-KIBALA, E., ‘Phosphate Ester Inhibitors Solve
Naphthenic Acid Corrosion Problems’,
Oil & Gas Journal
,
Vol. 92, Issue 9, (28 February 1994).
2. ZETLMEISEL, M. J., ‘Naphthenic Acid Corrosion and its Control’,
NACE 96, paper No. 218.
3. BARABANOVA, G. V., IVANOV, V. I., KOSSAOVA, L. V. and
AKIMOVA, N. S., ‘Use of esters of acids of phosphorus as
lubricity additives for high-temperature synthetic lubricating
oils’, Chemistry and Technology of fuels and Oils, Vol. 12,
Issue 5, (May 1976).
Figure 6.
Dynamic test conditions and results –
barrier persistency test.
Table 2.
Dynamic test results – tri-ester and mono-/di-ester dosage profile
Corrosion rate (mils per year)
ppm P
4 hours
12 hours
Mono-/di-ester Tri-ester
Mono-/di-ester Tri-ester
Blank
264
264
166
166
10
150
300
116
161
40
0
242
0
164
50
230
154
70
Did not run
2
100
0
0








